Medicine - Institutions > Army health reports and medical documents > Scientific memoirs by officers of the Medical and Sanitary Departments of the Government of India > Number 34 - Standards of the constituents of the urine and blood and the bearing of the metabolism of Bengalis on the problems of nutrition > Standards of the constituents of the urine and blood and the bearing of the metabolism of Bengalis on the problems of nutrition
(26) Page 18
Download files
Individual page:
Thumbnail gallery: Grid view | List view
18
The principle of the method is to find the particular dilution of a nor-
mal solution of sodium chloride, two volumes of which will exactly
cause hæmolysis of one volume of the blood. Let this dilution be
N/45; this is equivalent to a 0.130 per cent. solution NaCl.
Now, in a similar way the dilution of the serum with distilled water
necessary in order that two volumes of the diluted serum may
cause hæmolysis of one volume of blood is found.
Therefore we have for example:—
N/45 Serum/8 = 130% NaCl therefore Serum=1.040% NaCl.
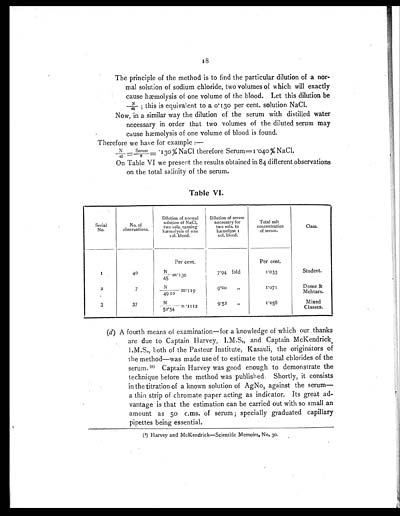
On Table VI we presert the results obtained in 84 different observations
on the total salinity of the serum.
Table VI.
| Serial No. |
No. of observations. |
Dilution of normal solution of NaCl, two vols. causing hæmolysis of one vol. blood. |
Dilution of serum necessary for two vols. to hæmolyse 1 vol. blood. |
Total salt concentration of serum. |
Class. |
| Per cent. | Per cent. | ||||
| 1 | 40 | N/45=.130 | 7.94 fold | 1.033 | Student. |
| 2 | 7 | N/4910=.119 | 9.00 " | 1.071 | Dome & Mehtars. |
| 3 | 37 | N/52.54=.1112 | 9.52 " | 1.058 | Mixed Classes. |
(d) A fourth means of examination—for a knowledge of which our thanks
are due to Captain Harvey, I.M.S., and Captain McKendrick,
I.M.S., both of the Pasteur Institute, Kasauli, the originators of
the method—was made use of to estimate the total chlorides of the
serum. (3) Captain Harvey was good enough to demonstrate the
technique before the method was published. Shortly, it consists
in the titration of a known solution of AgNo3 against the serum—
a thin strip of chromate paper acting as indicator. Its great ad-
vantage is that the estimation can be carried out with so small an
amount as 50 c.ms. of serum; specially graduated capillary
pipettes being essential.
(3) Harvey and McKendrick—Scientific Memoirs, No. 30.
Set display mode to: Large image | Zoom image | Transcription
Images and transcriptions on this page, including medium image downloads, may be used under the Creative Commons Attribution 4.0 International Licence unless otherwise stated. ![]()
| Permanent URL | https://digital.nls.uk/75032312 |
|---|