Encyclopaedia Britannica > Volume 21, ROT-Siam
(239) Page 229 - Salt
Download files
Complete book:
Individual page:
Thumbnail gallery: Grid view | List view
229
SALT
years greatly fallen off under the competition of the rock-
salt works of Cheshire, but some small manufactories still
exist, at North Shields and elsewhere, where salt is made
by dissolving rock-salt in sea water, and evaporating the
solution to crystallization by artificial heat.
The process of the spontaneous evaporation of sea water has
been very carefully studied by Usiglio on Mediterranean water at
Cette. The density at first was l-02. Primarily but a slight
deposit is formed (none until the concentration arrives at specific
gravity I’OSOQ), this deposit consisting for the most part of calcic
carbonate and ferric oxide. This goes on till a density of 1T315
is attained, when hydrated calcium sulphate begins to deposit, and
continues till specific gravity 1-2646 is reached. At a density of
I^IS the volume of the sea water has become reduced to T||-irths
of what it was at first, and from this moment the deposit becomes
augmented by sodium chloride, which goes down mixed with a
little magnesium chloride and sulphate. At specific gravity 1 '2461
a little sodium bromide has begun also to deposit. At specific
gravity 1’311 the volume of the water is only y-^-ths of what it
was at first, and it is thus composed:—
Magnesium sulphate 11‘45 per cent.
Magnesium chloride 19‘53 ,,
Sodium chloride 15-98 ,,
Sodium bromide 2 '04 ,,
Potassium chloride 3'30 ,,
Up to the time then that the water became concentrated to
specific gravity 1-218 only 0T50 of deposit had formed, and that
chiefly composed of lime and iron, but between specific gravity
I^IS and 1'313 there is deposited a mixture of—
Calcium sulphate 0-0283 percent.
Magnesium sulphate 0'0624 ,,
Magnesium chloride (P0153 ,,
Sodium chloride 27107 ,,
Sodium bromide 0,0222
2-8389
And of this we see that about 95 per cent, is sodium chloride.
Up to this point the separation of the salts has taken place in a
fairly regular manner, but now the temperature begins to exert an
influence, and some of the salts deposited in the cold of the night
dissolve again partially in the heat of the day. By night the
liquor gives nearly pure magnesium sulphate ; in the day the same
sulphate mixed with sodium and potassium chlorides is deposited.
The mother-liquor now falls a little in density to a specific gravity
of 1 -3082 to 1 '2965, and yields a very mixed deposit of magnesium
bromide and chloride, potassium chloride, and magnesium sulphate,
with the double magnesium and potassium sulphate, corresponding
to the kainite of Stassfurt. There is also deposited a double mag¬
nesium and potassium chloride, similar to the carnallite of Stassfurt,
and finally the mother-liquor, which has now again risen to specific
gravity 1-3374, contains only pure magnesium chloride.
The application of these results to the production of salt from sea
water is obvious. A large piece of land, varying from one or two
to several acres, barely above high-water mark, is levelled, and if
necessary puddled with clay so as to prevent the water from perco¬
lating and sinking away. In tidal seas a “jas” (as the storage
reservoir is called) is constructed alongside, similarly rendered im¬
pervious, in which the water is stored and allowed to settle and
concentrate to a certain extent. In non-tidal seas this storage
basin is not required. The prepared land is partitioned off into
large basins (adernes or muants) and others (called in France aires,
ceuillets, or tables salantes) which get smaller and more shallow in
proportion as they are intended to receive the water as it becomes
more and more concentrated, just sufficient fall being allowed from
one set of basins to the other to cause the water to flow slowly
through them. The flow is often assisted by pumping. The sea
salt thus made is collected into small heaps on the paths around
the basins or the floors of the basins themselves, and here it under¬
goes a first partial purification, the more deliquescent salts (espe¬
cially the magnesium chloride) being allowed to drain away. From
these heaps it is collected into larger ones, where it drains further,
and becomes more purified, Here it is protected by thatch till
required for sale.
The salt is collected from the surface by means of a sort of
wooden scoop or scraper which the workman pushes before him,
but in spite of every precaution some of the soil on which it is pro¬
duced is inevitably taken up with it, communicating a red or grey
tint. Sea salt is thence known in many of the French markets as
sel gris, and frequently contains as much as 15 per cent, of impurity.
Yet such is the ignorance and prejudice of many people that they
will buy it in preference to the purer article from the evaporation
of rock-salt brine, asserting its action to be milder and more even.
Even if this were true they forget that mud ought to be cheaper
than salt. The salt made on the coast of Brittany possesses the
following composition
Sodium chloride 87'97 per cent.
Magnesium chloride l-58 ,,
Magnesium sulphate 0 "50 ,,
Calcium sulphate l-65 ,,
Insoluble 0-80 ,,
W ater 7 "50 ,,
Generally speaking this salt goes into commerce just as it is,
but in some cases it is taken first to the refinery, where it either is
simply washed and then stove-dried before being sent out or is dis¬
solved in fresh water and then boiled down and crystallized like
white salt from rock-salt brine. The salt of the “ salines du midi ”
of the south-east of France is far purer than the above, however,
its composition being as follows :—
Sodium chloride 95-11 per cent.
Magnesium chloride 0"23 ,,
Magnesium sulphate 1-30 ,,
Calcium sulphate Q-91 ,,
Insoluble 0-10 ,’
Water 2'35 ’’
This is perhaps partly owing to the fact that of late years, by way
of obviating the above-mentioned cause of impurity, a species of
moss has been introduced there with some success from Portugal
and forms a bed on which the salt is deposited. The mother-
liquors from the crystallization of the common salt contain still a
little sodium chloride and most of the bromine and iodine of the
sea water, all the potassium salts, much magnesium sulphate, and
a large quantity of magnesium chloride. They are often thrown
away as useless, but lately, in the south of France, in the “salines
du midi,” they have been used for the production of certain chemi¬
cals by a system of ulterior treatment introduced by M. Merle and
still continued by his successor M. Pechinet.
As soon as the water arrives at specific gravity 1 -2407 and has
deposited most of its salt, it is drawn off' and stored in large tanks
of 50,000 or 60,000 cubic metres capacity. From these it is
withdrawn in successive portions, and artificially cooled to 0-4°
Fahr. Under these circumstances, indeed at any temperature
below 26° Fahr., a double decomposition takes place between the
sodium chloride and the magnesium sulphate—crystallized sodium
sulphate being thus separated. After being withdrawn and freed
from the mother-liquor by a hydro-extractor, this sulphate, which
contains two atoms of water, is then rendered anhydrous by heating
in a reverberatory furnace. From the refrigerating vessel the water-
now passes to an ordinary evaporating pan, where the remaining
salt is precipitated by boiling, collected, and purified by the hydro¬
extractor. Here the water attains a specific gravity 1 ‘2680, and,
being spread out in a thin layer on a smooth level bed of cement
or concrete, deposits on cooling all its potassium as the double
chloride of potassium and magnesium, the same as the carnallite of
Stassfurt.
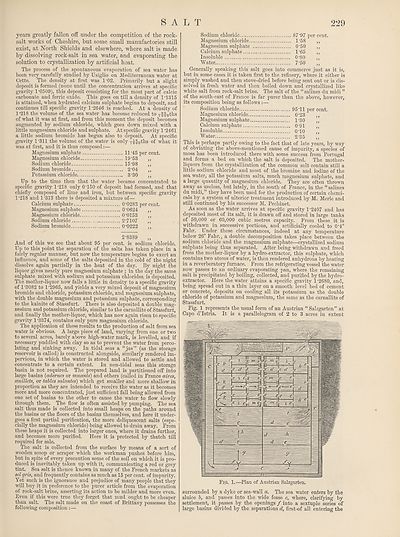
Fig. 1 represents the usual form of an Austrian “ Salzgarten” at
Capo dTstria. It is a parallelogram of 2 to 3 acres in extent
surrounded by a dyke or sea-wall a. The sea water enters by the
sluice b, and passes into the wide fosse c, where, clarifying by
settlement, it passes by the openings / into a sextuple series of
large basins divided by the separations d, first-of all entering the
SALT
years greatly fallen off under the competition of the rock-
salt works of Cheshire, but some small manufactories still
exist, at North Shields and elsewhere, where salt is made
by dissolving rock-salt in sea water, and evaporating the
solution to crystallization by artificial heat.
The process of the spontaneous evaporation of sea water has
been very carefully studied by Usiglio on Mediterranean water at
Cette. The density at first was l-02. Primarily but a slight
deposit is formed (none until the concentration arrives at specific
gravity I’OSOQ), this deposit consisting for the most part of calcic
carbonate and ferric oxide. This goes on till a density of 1T315
is attained, when hydrated calcium sulphate begins to deposit, and
continues till specific gravity 1-2646 is reached. At a density of
I^IS the volume of the sea water has become reduced to T||-irths
of what it was at first, and from this moment the deposit becomes
augmented by sodium chloride, which goes down mixed with a
little magnesium chloride and sulphate. At specific gravity 1 '2461
a little sodium bromide has begun also to deposit. At specific
gravity 1’311 the volume of the water is only y-^-ths of what it
was at first, and it is thus composed:—
Magnesium sulphate 11‘45 per cent.
Magnesium chloride 19‘53 ,,
Sodium chloride 15-98 ,,
Sodium bromide 2 '04 ,,
Potassium chloride 3'30 ,,
Up to the time then that the water became concentrated to
specific gravity 1-218 only 0T50 of deposit had formed, and that
chiefly composed of lime and iron, but between specific gravity
I^IS and 1'313 there is deposited a mixture of—
Calcium sulphate 0-0283 percent.
Magnesium sulphate 0'0624 ,,
Magnesium chloride (P0153 ,,
Sodium chloride 27107 ,,
Sodium bromide 0,0222
2-8389
And of this we see that about 95 per cent, is sodium chloride.
Up to this point the separation of the salts has taken place in a
fairly regular manner, but now the temperature begins to exert an
influence, and some of the salts deposited in the cold of the night
dissolve again partially in the heat of the day. By night the
liquor gives nearly pure magnesium sulphate ; in the day the same
sulphate mixed with sodium and potassium chlorides is deposited.
The mother-liquor now falls a little in density to a specific gravity
of 1 -3082 to 1 '2965, and yields a very mixed deposit of magnesium
bromide and chloride, potassium chloride, and magnesium sulphate,
with the double magnesium and potassium sulphate, corresponding
to the kainite of Stassfurt. There is also deposited a double mag¬
nesium and potassium chloride, similar to the carnallite of Stassfurt,
and finally the mother-liquor, which has now again risen to specific
gravity 1-3374, contains only pure magnesium chloride.
The application of these results to the production of salt from sea
water is obvious. A large piece of land, varying from one or two
to several acres, barely above high-water mark, is levelled, and if
necessary puddled with clay so as to prevent the water from perco¬
lating and sinking away. In tidal seas a “jas” (as the storage
reservoir is called) is constructed alongside, similarly rendered im¬
pervious, in which the water is stored and allowed to settle and
concentrate to a certain extent. In non-tidal seas this storage
basin is not required. The prepared land is partitioned off into
large basins (adernes or muants) and others (called in France aires,
ceuillets, or tables salantes) which get smaller and more shallow in
proportion as they are intended to receive the water as it becomes
more and more concentrated, just sufficient fall being allowed from
one set of basins to the other to cause the water to flow slowly
through them. The flow is often assisted by pumping. The sea
salt thus made is collected into small heaps on the paths around
the basins or the floors of the basins themselves, and here it under¬
goes a first partial purification, the more deliquescent salts (espe¬
cially the magnesium chloride) being allowed to drain away. From
these heaps it is collected into larger ones, where it drains further,
and becomes more purified, Here it is protected by thatch till
required for sale.
The salt is collected from the surface by means of a sort of
wooden scoop or scraper which the workman pushes before him,
but in spite of every precaution some of the soil on which it is pro¬
duced is inevitably taken up with it, communicating a red or grey
tint. Sea salt is thence known in many of the French markets as
sel gris, and frequently contains as much as 15 per cent, of impurity.
Yet such is the ignorance and prejudice of many people that they
will buy it in preference to the purer article from the evaporation
of rock-salt brine, asserting its action to be milder and more even.
Even if this were true they forget that mud ought to be cheaper
than salt. The salt made on the coast of Brittany possesses the
following composition
Sodium chloride 87'97 per cent.
Magnesium chloride l-58 ,,
Magnesium sulphate 0 "50 ,,
Calcium sulphate l-65 ,,
Insoluble 0-80 ,,
W ater 7 "50 ,,
Generally speaking this salt goes into commerce just as it is,
but in some cases it is taken first to the refinery, where it either is
simply washed and then stove-dried before being sent out or is dis¬
solved in fresh water and then boiled down and crystallized like
white salt from rock-salt brine. The salt of the “ salines du midi ”
of the south-east of France is far purer than the above, however,
its composition being as follows :—
Sodium chloride 95-11 per cent.
Magnesium chloride 0"23 ,,
Magnesium sulphate 1-30 ,,
Calcium sulphate Q-91 ,,
Insoluble 0-10 ,’
Water 2'35 ’’
This is perhaps partly owing to the fact that of late years, by way
of obviating the above-mentioned cause of impurity, a species of
moss has been introduced there with some success from Portugal
and forms a bed on which the salt is deposited. The mother-
liquors from the crystallization of the common salt contain still a
little sodium chloride and most of the bromine and iodine of the
sea water, all the potassium salts, much magnesium sulphate, and
a large quantity of magnesium chloride. They are often thrown
away as useless, but lately, in the south of France, in the “salines
du midi,” they have been used for the production of certain chemi¬
cals by a system of ulterior treatment introduced by M. Merle and
still continued by his successor M. Pechinet.
As soon as the water arrives at specific gravity 1 -2407 and has
deposited most of its salt, it is drawn off' and stored in large tanks
of 50,000 or 60,000 cubic metres capacity. From these it is
withdrawn in successive portions, and artificially cooled to 0-4°
Fahr. Under these circumstances, indeed at any temperature
below 26° Fahr., a double decomposition takes place between the
sodium chloride and the magnesium sulphate—crystallized sodium
sulphate being thus separated. After being withdrawn and freed
from the mother-liquor by a hydro-extractor, this sulphate, which
contains two atoms of water, is then rendered anhydrous by heating
in a reverberatory furnace. From the refrigerating vessel the water-
now passes to an ordinary evaporating pan, where the remaining
salt is precipitated by boiling, collected, and purified by the hydro¬
extractor. Here the water attains a specific gravity 1 ‘2680, and,
being spread out in a thin layer on a smooth level bed of cement
or concrete, deposits on cooling all its potassium as the double
chloride of potassium and magnesium, the same as the carnallite of
Stassfurt.
Fig. 1 represents the usual form of an Austrian “ Salzgarten” at
Capo dTstria. It is a parallelogram of 2 to 3 acres in extent
surrounded by a dyke or sea-wall a. The sea water enters by the
sluice b, and passes into the wide fosse c, where, clarifying by
settlement, it passes by the openings / into a sextuple series of
large basins divided by the separations d, first-of all entering the
Set display mode to:
![]() Universal Viewer |
Universal Viewer | ![]() Mirador |
Large image | Transcription
Mirador |
Large image | Transcription
Images and transcriptions on this page, including medium image downloads, may be used under the Creative Commons Attribution 4.0 International Licence unless otherwise stated. ![]()
| Encyclopaedia Britannica > Encyclopaedia Britannica > Volume 21, ROT-Siam > (239) Page 229 - Salt |
|---|
| Permanent URL | https://digital.nls.uk/193630386 |
|---|
| Attribution and copyright: |
|
|---|---|
| Shelfmark | EB.17 |
|---|---|
| Description | Ten editions of 'Encyclopaedia Britannica', issued from 1768-1903, in 231 volumes. Originally issued in 100 weekly parts (3 volumes) between 1768 and 1771 by publishers: Colin Macfarquhar and Andrew Bell (Edinburgh); editor: William Smellie: engraver: Andrew Bell. Expanded editions in the 19th century featured more volumes and contributions from leading experts in their fields. Managed and published in Edinburgh up to the 9th edition (25 volumes, from 1875-1889); the 10th edition (1902-1903) re-issued the 9th edition, with 11 supplementary volumes. |
|---|---|
| Additional NLS resources: |
|

