New volumes of the Encyclopædia Britannica > Volume 30, K-MOR
(321) Page 291
Download files
Complete book:
Individual page:
Thumbnail gallery: Grid view | List view
291
LIQUID GASES
was obtained corresponding to that given by other pure
metals. As to alloys, there is usually some definite
mixture of two pure metals which has a maximum resist¬
ivity, often greater than that of either of the constituents.
It appears too that high, if not the highest, resistivity
corresponds to possible chemical compounds of the two
metals employed, e.g., platinum 33 parts with silver 66
parts = PtAg4; iron 80 with nickel 20 = Fe^i; plati¬
num 80 with iridium 20 = IrPt4; and copper 70 with
manganese 30 = Cu2Mn. The product obtained by adding
a small quantity of one metal to another has a higher
specific resistance than the predominant constituent, but
the curve is parallel to, and therefore the same in shape
as, that of the latter (cf. the curves for various mixtures
of A1 and Cu on the chart). The behaviour of carbon
and of insulators like guttapercha, glass, ebonite, &c., is
in complete contrast to the metals, for their resistivity
steadily increases with cold. The thermoelectric properties
of metals at low temperatures are discussed in the article
Thermoelectricity.
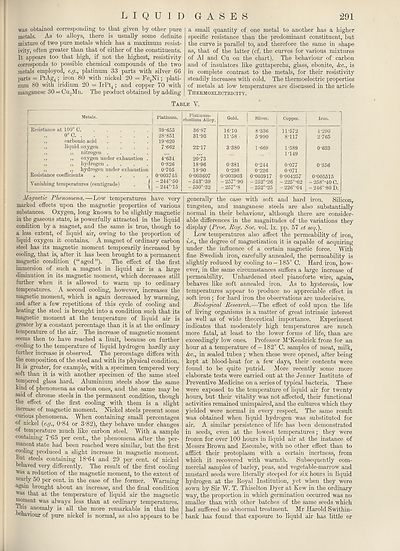
Table Y.
Metals.
Platinum.
Resistance at 100° C.
o°c. .
,, carbonic acid
,, liquid oxygen
,, ,, nitrogen
,, ,, oxygen under exhaustion
,, ,, hydrogen
,, ,, hydrogen under exhaustion
Resistance coefficients . . . . .
Vanishing temperatures (centigrade)
39-655
28-851
19-620
7-662
4-634
0-826
0-705
0-003745
-244°-50
-244°-15
Platinum-
rliodiuin Alloy.
36-87
31-93
22-17
20'-73
18-96
18-90
0-003607
- 543°"39
-530°-32
Gold.
Silver.
Copper.
16-10
11-58
3-380
0-381
0-298
0-003903
-257°-90
-257°-8
8-336
5-990
1-669
0-244
0-226
0-003917
-252°-26
-252°-25
11-572
8-117
1-589
1-149
0-077
0-071
0-004257
-225°-62
- 226°"04
4-290
2-765
0-633
0-356
0-005515
-258°-40 C.
-246°-80 D.
Magnetic Phenomena.—Low temperatures have very
marked effects upon the magnetic properties of various
substances. Oxygen, long known to be slightly magnetic
in the gaseous state, is powerfully attracted in the liquid
condition by a magnet, and the same is true, though to
a less extent, of liquid air, owing to the proportion of
liquid oxygen it contains. A magnet of ordinary carbon
steel has its magnetic moment temporarily increased by
cooling, that is, after it has been brought to a permanent
magnetic condition (“aged”). The effect of the first
immersion of such a magnet in liquid air is a large
diminution in its magnetic moment, which decreases still
further when it is allowed to warm up to ordinary
temperatures. A second cooling, however, increases the
magnetic moment, which is again decreased by warming,
and after a few repetitions of this cycle of cooling and
heating the steel is brought into a condition such that its
magnetic moment at the temperature of liquid air is
greater by a constant percentage than it is at the ordinary
temperature of the air. The increase of magnetic moment
seems then to have reached a limit, because on further
■cooling to the temperature of liquid hydrogen hardly any
further increase is observed. The percentage differs with
tlie composition of the steel and with its physical condition.
It is greater, for example, with a specimen tempered very
soft than it is with another specimen of the same steel
tempered glass hard. Aluminium steels show the same
kind of phenomena as carbon ones, and the same may be
said of chrome steels in the permanent condition, though
the effect of the first cooling with them is a slight
increase of magnetic moment. Nickel steels present some
curious phenomena. When containing small percentages
■of nickel {e.g., 0-84 or 3-82), they behave under changes
-of temperature much like carbon steel. With a sample
containing 7-65 per cent., the phenomena after the per¬
manent state had been reached were similar, but the first
cooling produced a slight increase in magnetic moment.
But steels containing 18-64 and 29 per cent, of nickel
behaved very differently. The result of the first cooling
was a reduction of the magnetic moment, to the extent of
nearly 50 per cent, in the case of the former. Warming
■again brought about an increase, and the final condition
was that at the temperature of liquid air the magnetic
nioment was always less than at ordinary temperatures.
Inis anomaly is all the more remarkable in that the
behaviour of pure nickel is normal, as also appears to be
generally the case with soft and hard iron. Silicon,
tungsten, and manganese steels are also substantially
normal in their behaviour, although there are consider¬
able differences in the magnitudes of the variations they
display {Proc. Roy. Soc. vol. lx. pp. 57 et seq.).
Low temperatures also affect the permeability of iron,
i.e., the degree of magnetization it is capable of acquiring
under the influence of a certain magnetic force. With
fine Swedish iron, carefully annealed, the permeability is
slightly reduced by cooling to - 185° C. Hard iron, how¬
ever, in the same circumstances suffers a large increase of
permeability. Unhardened steel pianoforte wire, again,
behaves like soft annealed iron. As to hysteresis, low
temperatures appear to produce no appreciable effect in
soft iron; for hard iron the observations are undecisive.
Biological Research.—The effect of cold upon the life
of living organisms is a matter of great intrinsic interest
as well as of wide theoretical importance. Experiment
indicates that moderately high temperatures are much
more fatal, at least to the lower forms of life, than are
exceedingly low ones. Professor JVFKendrick froze for an
hour at a temperature of - 182° C. samples of meat, milk,
etc., in sealed tubes; when these were opened, after being
kept at blood-heat for a few days, their contents were
found to be quite putrid. More recently some more
elaborate tests were carried out at the Jenner Institute of
Preventive Medicine on a series of typical bacteria. These
were exposed to the temperature of liquid air for twenty
hours, but their vitality was not affected, their functional
activities remained unimpaired, and the cultures which they
yielded were normal in every respect. The same result
was obtained when liquid hydrogen was substituted for
air. A similar persistence of life has been demonstrated
in seeds, even at the lowest temperatures; they were
frozen for over 100 hours in liquid air at the instance of
Messrs Brown and Escombe, with no other effect than to
afflict their protoplasm with a certain inertness, from
which it recovered with warmth. Subsequently com¬
mercial samples of barley, peas, and vegetable-marrow and
mustard seeds were literally steeped for six hours in liquid
hydrogen at the Royal Institution, yet when they were
sown by Sir W. T. Thiselton Dyer at Kew in the ordinary
way, the proportion in which germination occurred was no
smaller than with other batches of the same seeds which
had suffered no abnormal treatment. Mr Harold Swithin-
bank has found that exposure to liquid air has little or
LIQUID GASES
was obtained corresponding to that given by other pure
metals. As to alloys, there is usually some definite
mixture of two pure metals which has a maximum resist¬
ivity, often greater than that of either of the constituents.
It appears too that high, if not the highest, resistivity
corresponds to possible chemical compounds of the two
metals employed, e.g., platinum 33 parts with silver 66
parts = PtAg4; iron 80 with nickel 20 = Fe^i; plati¬
num 80 with iridium 20 = IrPt4; and copper 70 with
manganese 30 = Cu2Mn. The product obtained by adding
a small quantity of one metal to another has a higher
specific resistance than the predominant constituent, but
the curve is parallel to, and therefore the same in shape
as, that of the latter (cf. the curves for various mixtures
of A1 and Cu on the chart). The behaviour of carbon
and of insulators like guttapercha, glass, ebonite, &c., is
in complete contrast to the metals, for their resistivity
steadily increases with cold. The thermoelectric properties
of metals at low temperatures are discussed in the article
Thermoelectricity.
Table Y.
Metals.
Platinum.
Resistance at 100° C.
o°c. .
,, carbonic acid
,, liquid oxygen
,, ,, nitrogen
,, ,, oxygen under exhaustion
,, ,, hydrogen
,, ,, hydrogen under exhaustion
Resistance coefficients . . . . .
Vanishing temperatures (centigrade)
39-655
28-851
19-620
7-662
4-634
0-826
0-705
0-003745
-244°-50
-244°-15
Platinum-
rliodiuin Alloy.
36-87
31-93
22-17
20'-73
18-96
18-90
0-003607
- 543°"39
-530°-32
Gold.
Silver.
Copper.
16-10
11-58
3-380
0-381
0-298
0-003903
-257°-90
-257°-8
8-336
5-990
1-669
0-244
0-226
0-003917
-252°-26
-252°-25
11-572
8-117
1-589
1-149
0-077
0-071
0-004257
-225°-62
- 226°"04
4-290
2-765
0-633
0-356
0-005515
-258°-40 C.
-246°-80 D.
Magnetic Phenomena.—Low temperatures have very
marked effects upon the magnetic properties of various
substances. Oxygen, long known to be slightly magnetic
in the gaseous state, is powerfully attracted in the liquid
condition by a magnet, and the same is true, though to
a less extent, of liquid air, owing to the proportion of
liquid oxygen it contains. A magnet of ordinary carbon
steel has its magnetic moment temporarily increased by
cooling, that is, after it has been brought to a permanent
magnetic condition (“aged”). The effect of the first
immersion of such a magnet in liquid air is a large
diminution in its magnetic moment, which decreases still
further when it is allowed to warm up to ordinary
temperatures. A second cooling, however, increases the
magnetic moment, which is again decreased by warming,
and after a few repetitions of this cycle of cooling and
heating the steel is brought into a condition such that its
magnetic moment at the temperature of liquid air is
greater by a constant percentage than it is at the ordinary
temperature of the air. The increase of magnetic moment
seems then to have reached a limit, because on further
■cooling to the temperature of liquid hydrogen hardly any
further increase is observed. The percentage differs with
tlie composition of the steel and with its physical condition.
It is greater, for example, with a specimen tempered very
soft than it is with another specimen of the same steel
tempered glass hard. Aluminium steels show the same
kind of phenomena as carbon ones, and the same may be
said of chrome steels in the permanent condition, though
the effect of the first cooling with them is a slight
increase of magnetic moment. Nickel steels present some
curious phenomena. When containing small percentages
■of nickel {e.g., 0-84 or 3-82), they behave under changes
-of temperature much like carbon steel. With a sample
containing 7-65 per cent., the phenomena after the per¬
manent state had been reached were similar, but the first
cooling produced a slight increase in magnetic moment.
But steels containing 18-64 and 29 per cent, of nickel
behaved very differently. The result of the first cooling
was a reduction of the magnetic moment, to the extent of
nearly 50 per cent, in the case of the former. Warming
■again brought about an increase, and the final condition
was that at the temperature of liquid air the magnetic
nioment was always less than at ordinary temperatures.
Inis anomaly is all the more remarkable in that the
behaviour of pure nickel is normal, as also appears to be
generally the case with soft and hard iron. Silicon,
tungsten, and manganese steels are also substantially
normal in their behaviour, although there are consider¬
able differences in the magnitudes of the variations they
display {Proc. Roy. Soc. vol. lx. pp. 57 et seq.).
Low temperatures also affect the permeability of iron,
i.e., the degree of magnetization it is capable of acquiring
under the influence of a certain magnetic force. With
fine Swedish iron, carefully annealed, the permeability is
slightly reduced by cooling to - 185° C. Hard iron, how¬
ever, in the same circumstances suffers a large increase of
permeability. Unhardened steel pianoforte wire, again,
behaves like soft annealed iron. As to hysteresis, low
temperatures appear to produce no appreciable effect in
soft iron; for hard iron the observations are undecisive.
Biological Research.—The effect of cold upon the life
of living organisms is a matter of great intrinsic interest
as well as of wide theoretical importance. Experiment
indicates that moderately high temperatures are much
more fatal, at least to the lower forms of life, than are
exceedingly low ones. Professor JVFKendrick froze for an
hour at a temperature of - 182° C. samples of meat, milk,
etc., in sealed tubes; when these were opened, after being
kept at blood-heat for a few days, their contents were
found to be quite putrid. More recently some more
elaborate tests were carried out at the Jenner Institute of
Preventive Medicine on a series of typical bacteria. These
were exposed to the temperature of liquid air for twenty
hours, but their vitality was not affected, their functional
activities remained unimpaired, and the cultures which they
yielded were normal in every respect. The same result
was obtained when liquid hydrogen was substituted for
air. A similar persistence of life has been demonstrated
in seeds, even at the lowest temperatures; they were
frozen for over 100 hours in liquid air at the instance of
Messrs Brown and Escombe, with no other effect than to
afflict their protoplasm with a certain inertness, from
which it recovered with warmth. Subsequently com¬
mercial samples of barley, peas, and vegetable-marrow and
mustard seeds were literally steeped for six hours in liquid
hydrogen at the Royal Institution, yet when they were
sown by Sir W. T. Thiselton Dyer at Kew in the ordinary
way, the proportion in which germination occurred was no
smaller than with other batches of the same seeds which
had suffered no abnormal treatment. Mr Harold Swithin-
bank has found that exposure to liquid air has little or
Set display mode to:
![]() Universal Viewer |
Universal Viewer | ![]() Mirador |
Large image | Transcription
Mirador |
Large image | Transcription
Images and transcriptions on this page, including medium image downloads, may be used under the Creative Commons Attribution 4.0 International Licence unless otherwise stated. ![]()
| Encyclopaedia Britannica > New volumes of the Encyclopædia Britannica > Volume 30, K-MOR > (321) Page 291 |
|---|
| Permanent URL | https://digital.nls.uk/193571674 |
|---|
| Attribution and copyright: |
|
|---|---|
| Shelfmark | EB.18 |
|---|---|
| Description | Ten editions of 'Encyclopaedia Britannica', issued from 1768-1903, in 231 volumes. Originally issued in 100 weekly parts (3 volumes) between 1768 and 1771 by publishers: Colin Macfarquhar and Andrew Bell (Edinburgh); editor: William Smellie: engraver: Andrew Bell. Expanded editions in the 19th century featured more volumes and contributions from leading experts in their fields. Managed and published in Edinburgh up to the 9th edition (25 volumes, from 1875-1889); the 10th edition (1902-1903) re-issued the 9th edition, with 11 supplementary volumes. |
|---|---|
| Additional NLS resources: |
|

