Encyclopaedia Britannica > Volume 8, DIA-England
(645) Page 635
Download files
Complete book:
Individual page:
Thumbnail gallery: Grid view | List view
ELECTRICITY.
635
ienome- rays; that non-conductors will not thus phosphoresce at
mand all, or at least very imperfectly; and that most conduc-
Laws. tors give out no light whatever. Orpiment, and some of
the oxides of arsenic, tin, zinc, and lead, are exceptions
to this remark, and also the muriate of tin and the sul¬
phate and phosphate of lead. Non-conductors, and con¬
ductors which refuse to phosphoresce after feeble elec¬
trical discharges, become luminous after strong ones; while
imperfect conductors that phosphoresce after weak elec¬
trical discharges give no light whatever when the dis¬
charges are much increased. In support of the analogy
between electricity and phosphorescence, M. Dessaignes
remarks that phosphorescence is affected by the presence
of points. Fluor spar, which has the asperities produced
by fracture, phosphoresces readily, while the entire and
smooth crystal remains dark. The same is true of calca¬
reous spar, adularia, apatite, emerald, and common salt.
If both sides of the glass be rough, it phosphoresces
throughout; but if one side be rough and the other po¬
lished, it only shines when the rough surface receives the
heat. It is a fact still more curious, that when Iceland
crystal with smooth faces is exposed to the solar rays, it
acquires very little phosphorescence; whereas, if but one of
the faces is roughened and exposed to the sun, it readily
becomes luminous. In like manner, Arragonite becomes
luminous when a fractured face is exposed to the sun,
but acquires very little light when the smooth natural
surface is exposed to it. M. Dessaignes likewise maintains
that nearly all the bodies that are susceptible of phospho¬
rescence by friction become luminous by heat, by electri¬
city, and by exposure to light. The general view which
our author takes of these phenomena is, that phosphores¬
cence is produced by a particular fluid, which is set in
motion by light, by heat, by electricity, and by friction,
and that it is dissipated by overheating or too long expo¬
sure to light.
The influence of electricity upon phosphorescence and
li
the colours of certain bodies has been recently examined Phenome-
by Mr Pearsall of the Royal Institution, who has amply na and
confirmed the general result deduced experimentally by L Laws.
M. Dessaignes, that bodies which have lost their phos- A"*-0'"'/
phorescent property by calcination acquire it again when
an electrical discharge is passed through them. Having]yjr pear.
submitted a piece of chlorophane to a powerful heat, it sail,
gave out a strong phosphorescent light of a pale violet co¬
lour ; but the specimen decrepitated so much during its
calcination that a piece of sufficient size to be electrified
could not be preserved. He therefore placed the calcined
fragments in a glass tube, and sent through them three elec¬
trical discharges, the effect of which was the emission of a
deep violet light. He then heated the fragments upon pla¬
tinum, and they emitted a phosphoric light of different co¬
lours. Some of the fragments appeared green, others yel¬
low, and all of them finished by emitting a deep violet
light. These colours were evidently distinct from those
of the natural mineral, for a portion of the latter heated
at the same time produced only a feeble violet colour. Ano¬
ther portion of the same specimen, calcined but not elec¬
trified, emitted no light when heated.
A specimen of chlorophane, whose phosphorescence
had been destroyed by an intense heat, was exposed to
the solar rays for two days without any of its phospho¬
rescent quality being restored. A single electrical dis¬
charge, however, restored its phosphorescence, which in¬
creased in the ratio of the number and the intensity of
the shocks it received.- The green light emitted by the
action of heat was more deep and of longer continuance
after three, six, or even twelve discharges, than after one.
Mr Pearsall obtained the same results with apatite and
some diamonds; but electricity produced no effect in de¬
veloping phosphorescence by heat in amethyst, sapphire,
ruby, garnets, and other mineral substances which he
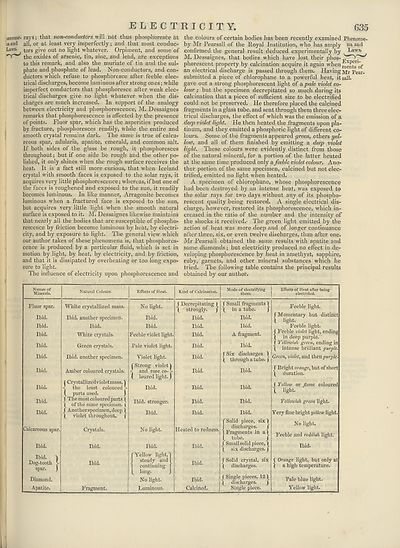
tried. The following table contains the principal results
obtained by our author.
Names of
Minerals.
Natural Colours.
Fluor spar.
Ibid.
Ibid.
Ibid.
Ibid.
Ibid.
Ibid.
Ibid.
Ibid.
Ibid.
Calcareous spar.
Ibid.
Ibid.
Dog-tooth
spar,
l- )
oth >
• )
Diamond.
Apatite.
White crystallized mass.
Ibid, another specimen.
Ibid.
White crystals.
Green crjrstals.
Ibid, another specimen.
Amber coloured crystals.
| Crystallizedvioletmass, I
i the least coloured k
( parts used. )
f The most coloured parts f
( of the same specimen. |
f Another specimen, deep )
( violet throughout. f
Crystals.
Ibid.
Ibid.
Fragment.
Effects of Heat.
No light.
Ibid.
Ibid.
Feeble violet light.
Pale violet light.
Violet light.
("Strong violet!
- y
and rose co.
( loured light.)
Ibid.
Ibid, stronger.
Ibid.
No light.
Ibid.
'Yellow light,'
steady and
continuing
_ long-
No light.
Luminous.
Kind of Calcination.
Mode of electrifying
them.
f Decrepitating |
\ strongly. j
Ibid.
Ibid.
Ibid.
f Small fragments)
( in a tube. j
Ibid.
Ibid.
Ibid.
Ibid.
Ibid.
Ibid.
Heated to redness,
Ibid.
Ibid.
Ibid.
Ibid.
A fragment.
Ibid.
( Six discharges
\ through a tube,
Ibid.
•}
Ibid.
Ibid.
Ibid.
fSolid piece, six)
discharges. f
Fragments in a|
f tube.
J Small solid piece, |
( six discharges.
j Solid crystal, six
( discharges.
Ibid.
Calcined.
( Single pieces, 12)
( discharges. J
Single piece.
Effects of Heat after being
electrified.
Feeble light.
f Momentary but distinct
t fight-
Feeble light.
J Feeble violet light, ending
( in deep purple,
f Yellowish green, ending in
( intense brilliant purple.
Green, violet, and then purple.
f Bright orange, but of short
\ duration.
j Yellow or jlame coloured
l light-
Yellowish green light.
Very fine bright ijellow light.
No light.
Feeble and reddish light.
Ibid.
Orange light, but only at
a high temperature.
Pale blue light.
Yellow light.
635
ienome- rays; that non-conductors will not thus phosphoresce at
mand all, or at least very imperfectly; and that most conduc-
Laws. tors give out no light whatever. Orpiment, and some of
the oxides of arsenic, tin, zinc, and lead, are exceptions
to this remark, and also the muriate of tin and the sul¬
phate and phosphate of lead. Non-conductors, and con¬
ductors which refuse to phosphoresce after feeble elec¬
trical discharges, become luminous after strong ones; while
imperfect conductors that phosphoresce after weak elec¬
trical discharges give no light whatever when the dis¬
charges are much increased. In support of the analogy
between electricity and phosphorescence, M. Dessaignes
remarks that phosphorescence is affected by the presence
of points. Fluor spar, which has the asperities produced
by fracture, phosphoresces readily, while the entire and
smooth crystal remains dark. The same is true of calca¬
reous spar, adularia, apatite, emerald, and common salt.
If both sides of the glass be rough, it phosphoresces
throughout; but if one side be rough and the other po¬
lished, it only shines when the rough surface receives the
heat. It is a fact still more curious, that when Iceland
crystal with smooth faces is exposed to the solar rays, it
acquires very little phosphorescence; whereas, if but one of
the faces is roughened and exposed to the sun, it readily
becomes luminous. In like manner, Arragonite becomes
luminous when a fractured face is exposed to the sun,
but acquires very little light when the smooth natural
surface is exposed to it. M. Dessaignes likewise maintains
that nearly all the bodies that are susceptible of phospho¬
rescence by friction become luminous by heat, by electri¬
city, and by exposure to light. The general view which
our author takes of these phenomena is, that phosphores¬
cence is produced by a particular fluid, which is set in
motion by light, by heat, by electricity, and by friction,
and that it is dissipated by overheating or too long expo¬
sure to light.
The influence of electricity upon phosphorescence and
li
the colours of certain bodies has been recently examined Phenome-
by Mr Pearsall of the Royal Institution, who has amply na and
confirmed the general result deduced experimentally by L Laws.
M. Dessaignes, that bodies which have lost their phos- A"*-0'"'/
phorescent property by calcination acquire it again when
an electrical discharge is passed through them. Having]yjr pear.
submitted a piece of chlorophane to a powerful heat, it sail,
gave out a strong phosphorescent light of a pale violet co¬
lour ; but the specimen decrepitated so much during its
calcination that a piece of sufficient size to be electrified
could not be preserved. He therefore placed the calcined
fragments in a glass tube, and sent through them three elec¬
trical discharges, the effect of which was the emission of a
deep violet light. He then heated the fragments upon pla¬
tinum, and they emitted a phosphoric light of different co¬
lours. Some of the fragments appeared green, others yel¬
low, and all of them finished by emitting a deep violet
light. These colours were evidently distinct from those
of the natural mineral, for a portion of the latter heated
at the same time produced only a feeble violet colour. Ano¬
ther portion of the same specimen, calcined but not elec¬
trified, emitted no light when heated.
A specimen of chlorophane, whose phosphorescence
had been destroyed by an intense heat, was exposed to
the solar rays for two days without any of its phospho¬
rescent quality being restored. A single electrical dis¬
charge, however, restored its phosphorescence, which in¬
creased in the ratio of the number and the intensity of
the shocks it received.- The green light emitted by the
action of heat was more deep and of longer continuance
after three, six, or even twelve discharges, than after one.
Mr Pearsall obtained the same results with apatite and
some diamonds; but electricity produced no effect in de¬
veloping phosphorescence by heat in amethyst, sapphire,
ruby, garnets, and other mineral substances which he
tried. The following table contains the principal results
obtained by our author.
Names of
Minerals.
Natural Colours.
Fluor spar.
Ibid.
Ibid.
Ibid.
Ibid.
Ibid.
Ibid.
Ibid.
Ibid.
Ibid.
Calcareous spar.
Ibid.
Ibid.
Dog-tooth
spar,
l- )
oth >
• )
Diamond.
Apatite.
White crystallized mass.
Ibid, another specimen.
Ibid.
White crystals.
Green crjrstals.
Ibid, another specimen.
Amber coloured crystals.
| Crystallizedvioletmass, I
i the least coloured k
( parts used. )
f The most coloured parts f
( of the same specimen. |
f Another specimen, deep )
( violet throughout. f
Crystals.
Ibid.
Ibid.
Fragment.
Effects of Heat.
No light.
Ibid.
Ibid.
Feeble violet light.
Pale violet light.
Violet light.
("Strong violet!
- y
and rose co.
( loured light.)
Ibid.
Ibid, stronger.
Ibid.
No light.
Ibid.
'Yellow light,'
steady and
continuing
_ long-
No light.
Luminous.
Kind of Calcination.
Mode of electrifying
them.
f Decrepitating |
\ strongly. j
Ibid.
Ibid.
Ibid.
f Small fragments)
( in a tube. j
Ibid.
Ibid.
Ibid.
Ibid.
Ibid.
Ibid.
Heated to redness,
Ibid.
Ibid.
Ibid.
Ibid.
A fragment.
Ibid.
( Six discharges
\ through a tube,
Ibid.
•}
Ibid.
Ibid.
Ibid.
fSolid piece, six)
discharges. f
Fragments in a|
f tube.
J Small solid piece, |
( six discharges.
j Solid crystal, six
( discharges.
Ibid.
Calcined.
( Single pieces, 12)
( discharges. J
Single piece.
Effects of Heat after being
electrified.
Feeble light.
f Momentary but distinct
t fight-
Feeble light.
J Feeble violet light, ending
( in deep purple,
f Yellowish green, ending in
( intense brilliant purple.
Green, violet, and then purple.
f Bright orange, but of short
\ duration.
j Yellow or jlame coloured
l light-
Yellowish green light.
Very fine bright ijellow light.
No light.
Feeble and reddish light.
Ibid.
Orange light, but only at
a high temperature.
Pale blue light.
Yellow light.
Set display mode to:
![]() Universal Viewer |
Universal Viewer | ![]() Mirador |
Large image | Transcription
Mirador |
Large image | Transcription
Images and transcriptions on this page, including medium image downloads, may be used under the Creative Commons Attribution 4.0 International Licence unless otherwise stated. ![]()
| Encyclopaedia Britannica > Encyclopaedia Britannica > Volume 8, DIA-England > (645) Page 635 |
|---|
| Permanent URL | https://digital.nls.uk/193331390 |
|---|
| Attribution and copyright: |
|
|---|
| Description | Ten editions of 'Encyclopaedia Britannica', issued from 1768-1903, in 231 volumes. Originally issued in 100 weekly parts (3 volumes) between 1768 and 1771 by publishers: Colin Macfarquhar and Andrew Bell (Edinburgh); editor: William Smellie: engraver: Andrew Bell. Expanded editions in the 19th century featured more volumes and contributions from leading experts in their fields. Managed and published in Edinburgh up to the 9th edition (25 volumes, from 1875-1889); the 10th edition (1902-1903) re-issued the 9th edition, with 11 supplementary volumes. |
|---|---|
| Additional NLS resources: |
|

