Encyclopaedia Britannica, or, a Dictionary of arts, sciences, and miscellaneous literature : enlarged and improved. Illustrated with nearly six hundred engravings > Volume 5, BUR-CHI
(472) Page 444
Download files
Complete book:
Individual page:
Thumbnail gallery: Grid view | List view
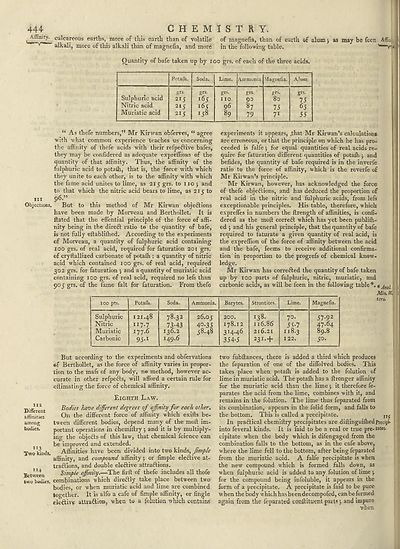
444 CHEMISTRY.
, Affinity- calcareous earths, more of this earth than of volatile of magnefia, than of earth ©f alum j as may be feen Affijj
alkali, more of this alkali than of magnefia, and more in the following table. W~Y'4
Quantity of bafe taken up by 100 grs. of each of the three acids.
Sulphuric acid
Nitric acid
Muriatic acid
Potafh.
.grs
215
2IJ
215
Soda.
grs.
I^S
165
158
Lime.
grs.
no
96
89
Ammonia
grs.
90
87
79
Vlagnefia.
grs.
80
75
71
Alum
grs.
75
65
55
“ As thefe numbers,” Mr Kirwan obferves, “ agree
with what common experience teaches us concerning
the affinity of thefe acids w ith their refpeftive bafes,
they may be confidered as adequate expreffions of the
quantity of that affinity. Thus, the affinity of the
fulphuric acid to potaffi, that is, the force with which
they unite to each other, is to the affinity with which
the fame acid unites to lime, as 215 grs. to no j and
to that which the nitric acid bears to lime, as 21 c to
tit 96.”
Objections. But to this method of Mr Kirwan obje&ions
have been made by Morveau and Berthollet. It is
ftated that the eflential principle of the force of affi¬
nity being in the direct ratio to the quantity of bafe,
is not fully eftabliffied. According to the experiments
of Morveau, a quantity of fulphuric acid containing
100 grs. of real acid, required for faturation 201 grs.
of cryfiallized carbonate of potafh : a quantity of nitric
acid which contained 100 grs. of real acid, required
302 grs. for faturation j and a quantity of muriatic acid
containing ico grs. of real acid, required no lefs than
9°5 grs. of the fame fait for faturation. From thefe
experiments it appears, .that Mr Kirwan’s calculations,
are erroneous, or that the principle on which he has pro¬
ceeded is falfe •, for equal quantities of real acids re¬
quire for faturation different quantities of potafh j and
befides, the quantity of bafe required is in the inverfe
ratio to the force of affinity, which is the reverfe of
Mr Kirwan’s principle.
Mr Kirwan, however, has acknowledged the force
of thefe objections, and has deduced the proportion of
real acid in the nitric and fulphuric acids, from lefs
exceptionable principles. His table, therefore, which
expreffes in numbers the ftrength of affinities, is confi¬
dered as the moff correCI which has yet been publifli-
ed $ and his general principle, that the quantity of bafe
required to faturate a given quantity of real acid, is
the expreffion of the force of affinity between the acid
and the bafe, feems to receive additional confirma¬
tion in proportion to the progrefs of chemical know¬
ledge.
Mr Kirwan has correCled the quantity of bafe taken
up by 100 parts of fulphuric, nitric, muriatic, and
carbonic acids, as will be feen in the following table*.#Anal
Min. Wt
ten.
100 pts.
Potafli.
Soda.
Ammonia.
Barytes.
Strontites.
Lime.
Magnefia.
Sulphuric
Nitric
Muriatic
Carbonic
121.48
117.7
177.6
95-1
78.32
73-43
136.2
149.6
26.05
4°-35
58.48
200.
178.12
314.46
354-5
138.
116.86
216.21
231- +
70.
55-7
118.3
122.
57-92
47.64
89.8
50.
But according to the experiments and obfervations
®f Berthollet, as the force of affinity varies in propor¬
tion to the mafs of any body, no method, however ac¬
curate in other refpe&s, will afford a certain rule for
eftimating the force of chemical affinity.
Eighth Law.
D fferent Bodies have different degrees of affinity for each other.
affinities On the different force of affinity which exifts be-
among tween different bodies, depend many of the moft im-
bodies. portant operations in chemiftry and it is by multiply¬
ing the objedls of this law, that chemical fcicnce can
be improved and extended.
Two kinds. Affinities have been divided into two kinds, Jitnple
' affinity, and compound affinity; or fimple elective at¬
tractions, and double eleCtive attractions.
Between Simple affinity.-—The firft of thefe includes all thofe
two badies. combinations which direCtly take place between two
bodies, or when muriatic acid and lime are combined
together. It is alfo a cafe of fimple affinity, or fingle
elective attraction, when to a Solution which contains
tw o fubftances, there is added a third which produces
the feparation of one of the diffolved bodies. This
takes place when potaffi is added to the folution of
lime in muriatic acid. The potaffi has a ftronger affinity
for the muriatic acid than the lime j it therefore fe-
parates the acid from the lime, combines with it, and
remains in the folution. The lime thus feparated from
its combination, appears in the folid form, and falls to
the bottom. This is called a precipitate. n5
In practical chemiftry precipitates are diftinguilhedprecipk
into feveral kinds. It is faid to be a real or true pre-tales,
cipitate when the body which is difengaged from the
combination falls to the bottom, as in the cafe above,
where the lime fell to the bottom, after being feparated
from the muriatic acid. A falfe precipitate is when
the new compound which is formed falls down, as
when fulphuric acid is added to any folution of lime \
for the compound being infoluble, it appears in the
form of a precipitate. A precipitate is faid to be pure
when the body which hasbeendecompofed, can be formed
again from the feparated conftituent parts 5 and impure
when
, Affinity- calcareous earths, more of this earth than of volatile of magnefia, than of earth ©f alum j as may be feen Affijj
alkali, more of this alkali than of magnefia, and more in the following table. W~Y'4
Quantity of bafe taken up by 100 grs. of each of the three acids.
Sulphuric acid
Nitric acid
Muriatic acid
Potafh.
.grs
215
2IJ
215
Soda.
grs.
I^S
165
158
Lime.
grs.
no
96
89
Ammonia
grs.
90
87
79
Vlagnefia.
grs.
80
75
71
Alum
grs.
75
65
55
“ As thefe numbers,” Mr Kirwan obferves, “ agree
with what common experience teaches us concerning
the affinity of thefe acids w ith their refpeftive bafes,
they may be confidered as adequate expreffions of the
quantity of that affinity. Thus, the affinity of the
fulphuric acid to potaffi, that is, the force with which
they unite to each other, is to the affinity with which
the fame acid unites to lime, as 215 grs. to no j and
to that which the nitric acid bears to lime, as 21 c to
tit 96.”
Objections. But to this method of Mr Kirwan obje&ions
have been made by Morveau and Berthollet. It is
ftated that the eflential principle of the force of affi¬
nity being in the direct ratio to the quantity of bafe,
is not fully eftabliffied. According to the experiments
of Morveau, a quantity of fulphuric acid containing
100 grs. of real acid, required for faturation 201 grs.
of cryfiallized carbonate of potafh : a quantity of nitric
acid which contained 100 grs. of real acid, required
302 grs. for faturation j and a quantity of muriatic acid
containing ico grs. of real acid, required no lefs than
9°5 grs. of the fame fait for faturation. From thefe
experiments it appears, .that Mr Kirwan’s calculations,
are erroneous, or that the principle on which he has pro¬
ceeded is falfe •, for equal quantities of real acids re¬
quire for faturation different quantities of potafh j and
befides, the quantity of bafe required is in the inverfe
ratio to the force of affinity, which is the reverfe of
Mr Kirwan’s principle.
Mr Kirwan, however, has acknowledged the force
of thefe objections, and has deduced the proportion of
real acid in the nitric and fulphuric acids, from lefs
exceptionable principles. His table, therefore, which
expreffes in numbers the ftrength of affinities, is confi¬
dered as the moff correCI which has yet been publifli-
ed $ and his general principle, that the quantity of bafe
required to faturate a given quantity of real acid, is
the expreffion of the force of affinity between the acid
and the bafe, feems to receive additional confirma¬
tion in proportion to the progrefs of chemical know¬
ledge.
Mr Kirwan has correCled the quantity of bafe taken
up by 100 parts of fulphuric, nitric, muriatic, and
carbonic acids, as will be feen in the following table*.#Anal
Min. Wt
ten.
100 pts.
Potafli.
Soda.
Ammonia.
Barytes.
Strontites.
Lime.
Magnefia.
Sulphuric
Nitric
Muriatic
Carbonic
121.48
117.7
177.6
95-1
78.32
73-43
136.2
149.6
26.05
4°-35
58.48
200.
178.12
314.46
354-5
138.
116.86
216.21
231- +
70.
55-7
118.3
122.
57-92
47.64
89.8
50.
But according to the experiments and obfervations
®f Berthollet, as the force of affinity varies in propor¬
tion to the mafs of any body, no method, however ac¬
curate in other refpe&s, will afford a certain rule for
eftimating the force of chemical affinity.
Eighth Law.
D fferent Bodies have different degrees of affinity for each other.
affinities On the different force of affinity which exifts be-
among tween different bodies, depend many of the moft im-
bodies. portant operations in chemiftry and it is by multiply¬
ing the objedls of this law, that chemical fcicnce can
be improved and extended.
Two kinds. Affinities have been divided into two kinds, Jitnple
' affinity, and compound affinity; or fimple elective at¬
tractions, and double eleCtive attractions.
Between Simple affinity.-—The firft of thefe includes all thofe
two badies. combinations which direCtly take place between two
bodies, or when muriatic acid and lime are combined
together. It is alfo a cafe of fimple affinity, or fingle
elective attraction, when to a Solution which contains
tw o fubftances, there is added a third which produces
the feparation of one of the diffolved bodies. This
takes place when potaffi is added to the folution of
lime in muriatic acid. The potaffi has a ftronger affinity
for the muriatic acid than the lime j it therefore fe-
parates the acid from the lime, combines with it, and
remains in the folution. The lime thus feparated from
its combination, appears in the folid form, and falls to
the bottom. This is called a precipitate. n5
In practical chemiftry precipitates are diftinguilhedprecipk
into feveral kinds. It is faid to be a real or true pre-tales,
cipitate when the body which is difengaged from the
combination falls to the bottom, as in the cafe above,
where the lime fell to the bottom, after being feparated
from the muriatic acid. A falfe precipitate is when
the new compound which is formed falls down, as
when fulphuric acid is added to any folution of lime \
for the compound being infoluble, it appears in the
form of a precipitate. A precipitate is faid to be pure
when the body which hasbeendecompofed, can be formed
again from the feparated conftituent parts 5 and impure
when
Set display mode to:
![]() Universal Viewer |
Universal Viewer | ![]() Mirador |
Large image | Transcription
Mirador |
Large image | Transcription
Images and transcriptions on this page, including medium image downloads, may be used under the Creative Commons Attribution 4.0 International Licence unless otherwise stated. ![]()
| Permanent URL | https://digital.nls.uk/192990405 |
|---|
| Attribution and copyright: |
|
|---|
| Description | Ten editions of 'Encyclopaedia Britannica', issued from 1768-1903, in 231 volumes. Originally issued in 100 weekly parts (3 volumes) between 1768 and 1771 by publishers: Colin Macfarquhar and Andrew Bell (Edinburgh); editor: William Smellie: engraver: Andrew Bell. Expanded editions in the 19th century featured more volumes and contributions from leading experts in their fields. Managed and published in Edinburgh up to the 9th edition (25 volumes, from 1875-1889); the 10th edition (1902-1903) re-issued the 9th edition, with 11 supplementary volumes. |
|---|---|
| Additional NLS resources: |
|

