Encyclopaedia Britannica > Volume 15, PLA-RAM
(133) Page 117
Download files
Complete book:
Individual page:
Thumbnail gallery: Grid view | List view
i ft Set.
D
1,000
1,100
1)24-2
1,375
M71
1,692
l»833
2, coo
2,288
2.444
3’T43
3,666
4,coo
4>444
4,8a8
5»500
5,882
E
I ,coo
p
24 Set.
N E U
3<1 Set.
M
D
E
1,000
»i94
1 >375
i»°93 J !>23^
1,211
1,284
1*5 >9
1,669
1,796
^958
2,130
2,37*
2,936
3*391
3,706
4 °3>
4’43^
4,922
5,522
1
r
1,466
i»57'
1,692
2,000
2,444
1,000
1,224
1,288
i>3?2
1,41^
'>5l5
1,647
1,964
2.392
3>T 43 ! 3-°78
3,6^6 13,575
4.444 4.320
5,500.5,096
7.333 6>694
D
l,OCO
1,091
1,200
I»333
1,500
i.7H
2,000
2,400
3,000
4,000
6,coo
8,000
E
1,000
1,076
1,183
1>472
1,659
1,900
2,241
2.793
3.631
5.297
6,835
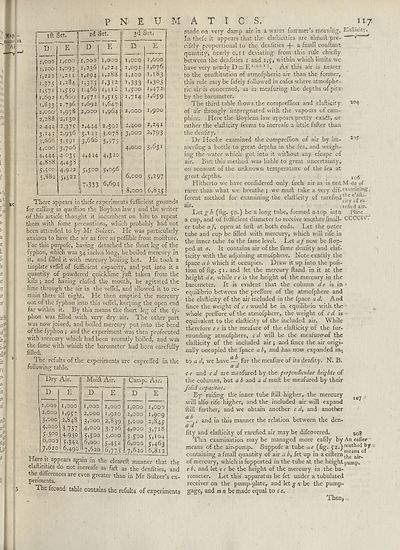
Tlierc appears in thefe experiments fulFicient grounds
for calling in queftion the Boylean law; and the writer
of this article thought it incumbent on him to repeat
them with fome precautions, which probably had not
been attended to by Mr Sulzer. He was particularly
anxious to have the air as free as poffible from moifture.
For tills purpofe, having detached the fhort leg of the
fyphon, which was 34 inches long, he boiled mercury in
it, and filled it with mercury boiling hot. He took a
tinplate veffel of fufficient capacity, and put into it a
quantity of powdered quicklime juft taken from the
kiln ; and having clofed tire mouth, he agitated the
lime through the air in the veffel, and allowed it to re¬
main there all night. He then emptied the mercury
out of the fyphon into this veffel, keeping the open end
far within it. By this means the fhort leg of the fy¬
phon was filled with very dry air. The other part
was now joined, and boiled mercury put into the bend
of the fyphon ; and the experiment was then profecuted
with mercury which had been recently boiled, and wa's
the fame with which the barometer had been carefully
filled.
The refults of the experiments are expreffed in the
followinrr table.
Dry Air.
D
1,003
2,000
3,000
4,000
5,500
6,003
7,620
E
1,000
‘.957
2,848
3 737
4.950
5.342
< 6,490
Moift Air.
D
1,000
2,000
3,000
4,000
5,500
6,000
7,620
1,000
1,920
2,839
3,726
5,000
5,452
6,775
Camp. Air.
D
1,000
2,000
3,000
4,000
1,000
1,909
2,845
3>7I8
5 500 I 5>104
6,000 : 5,463
7,620 I 6,812
^wi-e — aPPears again in the cleareft manner that the
dafticities do not increafe as fall as the denfities, and
the differences are even greater than in Mr Sulzer’s ex¬
periments.
The fecand table contains the refults of experiment;
A T I C S. 117
made on very damp air in a warm fummev’s morning. EMicity.
In thefe it appears that the elafticities are almofl pre- v-—'
cifely proportional to the denfities -f- a fmall conftant
quantity, nearly o, 1 x deviating from this rule chiefly
between the denfities 1 and 1,5, within which limits we
have very nearly D=.E100 1T As this air is nearer
to the confutation of atmofpheric air than the former,
this rule may be fafely followed in cafes where atmofphe¬
ric air is concerned, as in meafuring the depths of pits -
by the barometer.
The third table fhows the compreffion and elafticity 204
of air ftrongly impregnated with the vapours of cam-
phire. Here the Boylean law appears pretty exa£t, or
rather the elafticity feems to increafe a little fafter than
the denfity.
Dr Hooke examined the comprefiion of air by im- 2:>5
merfing a bottle to great depths in the fea, and weigh¬
ing the water which, got into it without any efcape of
air. But this method was liable to great uncertainty,
on account of the unknown temperature of the fea at
great depths. . ,o(5
Hitherto we have confidered only fuch air as is notM. deof
rarer than what we breathe ; we muft take a Very dif-exaniinin£-
ferent method for examining the elafticity of rarefied1
alr- _ refied air.
Let^ i (fig. 50.) be a long tube, formed a-top into Plate
a cup, and of fulficient diameter to receive another fmall- CCCCIV’.'’
er tube a /, open at firft at both ends. Let the outer
tube and cup be filled with mercury, which will rife in
the inner tube to the fame level. Let a f now be flop¬
ped at a. It contains air of the fame denfity and elaf¬
ticity with the adjoining atmofphere. Note exadtly the
{pace a b which it occupies. Draw it up into the pofi-
tion of fig. 51 • and let the mercury Hand in it at the
height de, while ce is the height of the mercury in the
barometer. It is evident that the column de is in >
equilibrio between the' preffure of the atmofphere and
the elallicity of the air included in the fpace a d. And
fince the weight oi c e would be in equilibrio with the
whole preffure of the atmofphere, the weight oi c d is --
equivalent to the elafticity of the included air. While
therefore r e is the meafure of the elafticity of the fur¬
rounding atmofphere, c d will be the meafure-of the
elafticity of the included air ; and fince the air origi¬
nally occupied the fpace a br and has now expanded in¬
to a dy we have — for the meafure of its denfity. N. B.
a d
c e and c d are meafured by the perpendicular heights of
the columns, but a b and a d mull be meafured by their
folid capacities.
By railing the inner tube flill higher, the mercury _
will alfo rife higher, and the included air will expand
ft ill farther, and we obtain another c dy and another
-- ; and in this manner the relation between the den-
a d
fity and elafticity of rarefied air may be difeovered. a0S
This examination may be managed more eafily by An eafier
means of the air-pump. Suppofe a tube a e (fig. 52.) met^0<^8?
containing a fmall quantity of air a by fet up in a ciftern
of mercury, which is fupported in the tube at the height ^ump.
e b, and let t c be the height of the mercury in the ba¬
rometer. Let this apparatus be fet under a tubulated
receiver on the pump-plate, and let £ « be the pump-
gage, and « be made equal to c e.
Then,
D
1,000
1,100
1)24-2
1,375
M71
1,692
l»833
2, coo
2,288
2.444
3’T43
3,666
4,coo
4>444
4,8a8
5»500
5,882
E
I ,coo
p
24 Set.
N E U
3<1 Set.
M
D
E
1,000
»i94
1 >375
i»°93 J !>23^
1,211
1,284
1*5 >9
1,669
1,796
^958
2,130
2,37*
2,936
3*391
3,706
4 °3>
4’43^
4,922
5,522
1
r
1,466
i»57'
1,692
2,000
2,444
1,000
1,224
1,288
i>3?2
1,41^
'>5l5
1,647
1,964
2.392
3>T 43 ! 3-°78
3,6^6 13,575
4.444 4.320
5,500.5,096
7.333 6>694
D
l,OCO
1,091
1,200
I»333
1,500
i.7H
2,000
2,400
3,000
4,000
6,coo
8,000
E
1,000
1,076
1,183
1>472
1,659
1,900
2,241
2.793
3.631
5.297
6,835
Tlierc appears in thefe experiments fulFicient grounds
for calling in queftion the Boylean law; and the writer
of this article thought it incumbent on him to repeat
them with fome precautions, which probably had not
been attended to by Mr Sulzer. He was particularly
anxious to have the air as free as poffible from moifture.
For tills purpofe, having detached the fhort leg of the
fyphon, which was 34 inches long, he boiled mercury in
it, and filled it with mercury boiling hot. He took a
tinplate veffel of fufficient capacity, and put into it a
quantity of powdered quicklime juft taken from the
kiln ; and having clofed tire mouth, he agitated the
lime through the air in the veffel, and allowed it to re¬
main there all night. He then emptied the mercury
out of the fyphon into this veffel, keeping the open end
far within it. By this means the fhort leg of the fy¬
phon was filled with very dry air. The other part
was now joined, and boiled mercury put into the bend
of the fyphon ; and the experiment was then profecuted
with mercury which had been recently boiled, and wa's
the fame with which the barometer had been carefully
filled.
The refults of the experiments are expreffed in the
followinrr table.
Dry Air.
D
1,003
2,000
3,000
4,000
5,500
6,003
7,620
E
1,000
‘.957
2,848
3 737
4.950
5.342
< 6,490
Moift Air.
D
1,000
2,000
3,000
4,000
5,500
6,000
7,620
1,000
1,920
2,839
3,726
5,000
5,452
6,775
Camp. Air.
D
1,000
2,000
3,000
4,000
1,000
1,909
2,845
3>7I8
5 500 I 5>104
6,000 : 5,463
7,620 I 6,812
^wi-e — aPPears again in the cleareft manner that the
dafticities do not increafe as fall as the denfities, and
the differences are even greater than in Mr Sulzer’s ex¬
periments.
The fecand table contains the refults of experiment;
A T I C S. 117
made on very damp air in a warm fummev’s morning. EMicity.
In thefe it appears that the elafticities are almofl pre- v-—'
cifely proportional to the denfities -f- a fmall conftant
quantity, nearly o, 1 x deviating from this rule chiefly
between the denfities 1 and 1,5, within which limits we
have very nearly D=.E100 1T As this air is nearer
to the confutation of atmofpheric air than the former,
this rule may be fafely followed in cafes where atmofphe¬
ric air is concerned, as in meafuring the depths of pits -
by the barometer.
The third table fhows the compreffion and elafticity 204
of air ftrongly impregnated with the vapours of cam-
phire. Here the Boylean law appears pretty exa£t, or
rather the elafticity feems to increafe a little fafter than
the denfity.
Dr Hooke examined the comprefiion of air by im- 2:>5
merfing a bottle to great depths in the fea, and weigh¬
ing the water which, got into it without any efcape of
air. But this method was liable to great uncertainty,
on account of the unknown temperature of the fea at
great depths. . ,o(5
Hitherto we have confidered only fuch air as is notM. deof
rarer than what we breathe ; we muft take a Very dif-exaniinin£-
ferent method for examining the elafticity of rarefied1
alr- _ refied air.
Let^ i (fig. 50.) be a long tube, formed a-top into Plate
a cup, and of fulficient diameter to receive another fmall- CCCCIV’.'’
er tube a /, open at firft at both ends. Let the outer
tube and cup be filled with mercury, which will rife in
the inner tube to the fame level. Let a f now be flop¬
ped at a. It contains air of the fame denfity and elaf¬
ticity with the adjoining atmofphere. Note exadtly the
{pace a b which it occupies. Draw it up into the pofi-
tion of fig. 51 • and let the mercury Hand in it at the
height de, while ce is the height of the mercury in the
barometer. It is evident that the column de is in >
equilibrio between the' preffure of the atmofphere and
the elallicity of the air included in the fpace a d. And
fince the weight oi c e would be in equilibrio with the
whole preffure of the atmofphere, the weight oi c d is --
equivalent to the elafticity of the included air. While
therefore r e is the meafure of the elafticity of the fur¬
rounding atmofphere, c d will be the meafure-of the
elafticity of the included air ; and fince the air origi¬
nally occupied the fpace a br and has now expanded in¬
to a dy we have — for the meafure of its denfity. N. B.
a d
c e and c d are meafured by the perpendicular heights of
the columns, but a b and a d mull be meafured by their
folid capacities.
By railing the inner tube flill higher, the mercury _
will alfo rife higher, and the included air will expand
ft ill farther, and we obtain another c dy and another
-- ; and in this manner the relation between the den-
a d
fity and elafticity of rarefied air may be difeovered. a0S
This examination may be managed more eafily by An eafier
means of the air-pump. Suppofe a tube a e (fig. 52.) met^0<^8?
containing a fmall quantity of air a by fet up in a ciftern
of mercury, which is fupported in the tube at the height ^ump.
e b, and let t c be the height of the mercury in the ba¬
rometer. Let this apparatus be fet under a tubulated
receiver on the pump-plate, and let £ « be the pump-
gage, and « be made equal to c e.
Then,
Set display mode to:
![]() Universal Viewer |
Universal Viewer | ![]() Mirador |
Large image | Transcription
Mirador |
Large image | Transcription
Images and transcriptions on this page, including medium image downloads, may be used under the Creative Commons Attribution 4.0 International Licence unless otherwise stated. ![]()
| Encyclopaedia Britannica > Encyclopaedia Britannica > Volume 15, PLA-RAM > (133) Page 117 |
|---|
| Permanent URL | https://digital.nls.uk/191902505 |
|---|
| Attribution and copyright: |
|
|---|
| Description | Ten editions of 'Encyclopaedia Britannica', issued from 1768-1903, in 231 volumes. Originally issued in 100 weekly parts (3 volumes) between 1768 and 1771 by publishers: Colin Macfarquhar and Andrew Bell (Edinburgh); editor: William Smellie: engraver: Andrew Bell. Expanded editions in the 19th century featured more volumes and contributions from leading experts in their fields. Managed and published in Edinburgh up to the 9th edition (25 volumes, from 1875-1889); the 10th edition (1902-1903) re-issued the 9th edition, with 11 supplementary volumes. |
|---|---|
| Additional NLS resources: |
|

